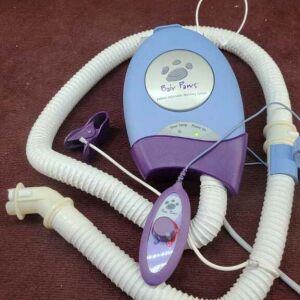
Description
RF Generator
The RF generator is designed to be used only with the Adiana Catheter. It is supplied with a connector cable (for attachment to the catheter) and a power cord. An optional footswitch accessory allows hands-free operation of the RF generator.
The RF generator is a microprocessor-controlled, bipolar, radiofrequency generator with automatic temperature control and a tissue contact sensor. It uses a menu-driven display to guide the operator through the procedure.
The RF generator provides continuous monitoring of catheter signals for determining proper catheter positioning, controlling lesion creation, ensuring matrix delivery and detecting error conditions.
There are no user-selectable controls for RF output, treatment time or treatment temperature. RF settings have been programmed in the generator software to ensure that the specified treatment temperature is achieved and maintained for the specified treatment duration. If necessary, the clinician can terminate treatment; however, no other physician control of output power is possible.
Indications for use
Adiana Permanent Contraception is indicated for women who desire permanent birth control (female sterilization) by occlusion of the fallopian tubes.
Contraindications
The Adiana System should not be used in a patient who:
-
Is uncertain about her desire to end fertility
-
Has clinical evidence of an active pelvic infection or history of a recent pelvic infection
-
Has intra-uterine pathology which would prevent access to either tubal ostium or the intramural portion of either fallopian tube (such as large submucous fibroids, uterine adhesions, apparent uni- or bilateral proximal tubal occlusion, suspected unicornuate uterus,etc.)
-
Is pregnant or suspects pregnancy
-
Is currently less than three months since her last pregnancy
-
Has previously undergone a tubal ligation
-
Is currently taking immunosuppressive medication(e.g., steroids)
-
Has a known allergy to contrast media
-
Patients must use alternative contraception for at least three months post treatment and until bilateral tubal occlusion is confirmed by HSG.
-
The Adiana procedure should be considered irreversible. There are no data on the safety or effectiveness or reversing the procedure through surgery.
-
The Adiana pivotal clinical trial effectiveness rates were based on women in whom bilateral placement was achieved. Effectiveness has not been determined for women with unilateral placement in a unicornuate uterus or with presumed or confirmed contralateral proximal tubal occlusion.
-
The safety and effectiveness of this procedure have not been demonstrated in patients under the age of 1 8 or over the age of 45.
-
Women who undergo sterilization at a relatively young age are at greater risk of regretting their decision.
-
Do not perform an endometrial ablation procedure concomitantly with the Adiana RF treatment and matrix placement procedures. Ablation may cause intrauterine synechiae, which could compromise the results of the three-month Adian HSG. If bilateral tubal occlusion is not confirmed during this HSG, the patient cannot rely on Adiana Permanent Contraception for pregnancy prevention.