Description
Introduction
The portable Implant Control System ICS 3000 is intended for use as a programming and monitoring system in the implantation and follow-up of electrotherapeutic implants.
It is a compact unit with numerous functions: … for clinical follow up of pacemakers, ICD, and CRT
Programmer … for monitoring the pacing function of pacemakers
Miniclinic … for real-time display and printing out up to 3 ECG
ECG printer and ECG monitor derivations – surface ECG (Einthoven) – esophageal
lead and up to 3 intracardiac leads.
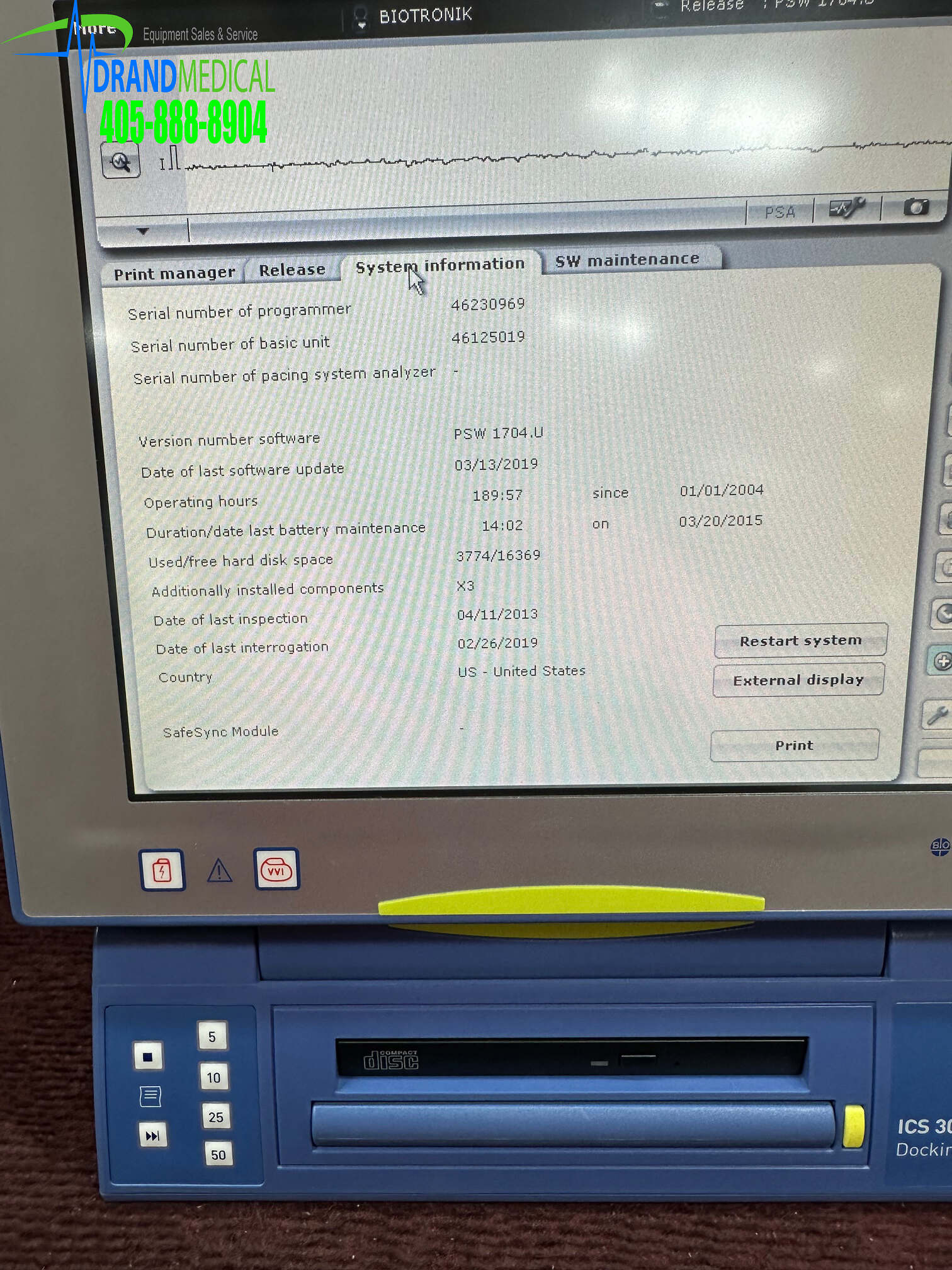
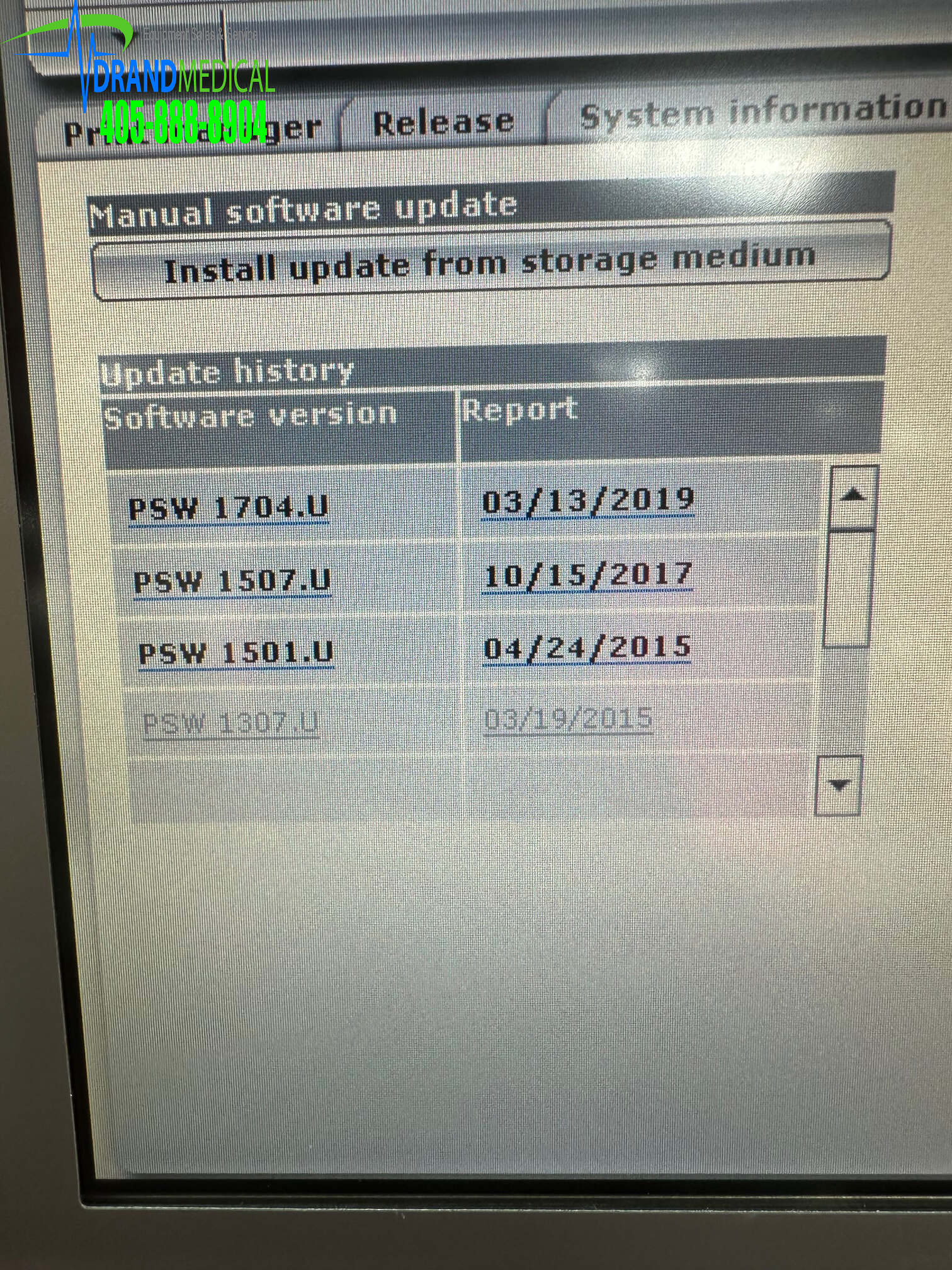
Data transfer… for transferring program data and the contents of the diagnostic memory for the purposes of computerized archiving and evaluation with the CDM 3000 Cardiac Data Manager.
Documentation … for generating follow-up reports using the integrated system printer and/or an external printer.
The system is modular and can be configured and expanded as required. The basic configuration consists of the following modules:
ICS 3000 DS Docking Station
ICS 3000 OM Operation Module
ICS 3000 PGH Programming Head
ICS 3000 SW Software for programmer, implant programs
Intended Use
The ICS 3000 is intended for use by physicians and trained medical personnel. To use the system, individuals must have a fundamental medical understanding of the respective therapy and detailed knowledge of how the implant functions and the conditions for its use. The operator should be present at all times when the ICS 3000 is in use.
Residual risk- No risks are associated with the programming system if it is used correctly and has been serviced and inspected according to BIOTRONIK specifications. The risk evaluation by the Risk Management Team has determined that the residual risk is as low as
reasonably possible.
Safety Instructions
The ECG connection makes electrical contact with the patient via the electrodes. It is an applied BF-type part and is defibrillation-proof when the approved patient cable is used