Description
System Description
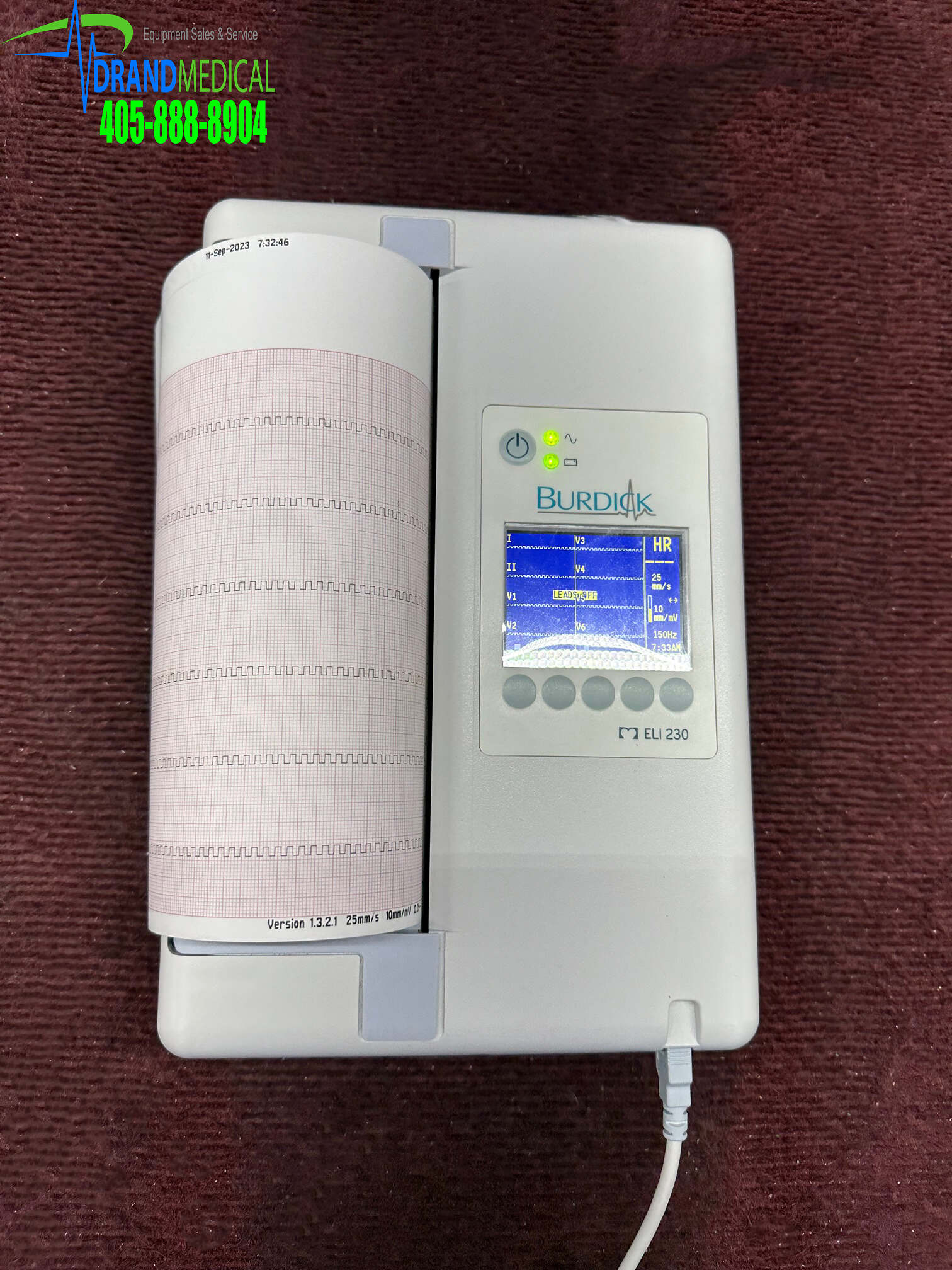
ELI 230 is a 12-lead diagnostic electrocardiograph used for acquiring, viewing, and printing of adult and pediatric 12-lead ECG data. The device is optionally equipped with Welch Allyn’s VERITAS™ resting ECG interpretation algorithm with age and gender specific criteria. If this option is enabled, the VERITAS algorithm can provide an over-reading physician with a silent second opinion through diagnostic statements output on the ECG report. For additional information on the VERITAS algorithm, please refer to the Physician’s Guide to Adult and Pediatric Resting ECG Interpretation. (See Accessories.)
Supported print formats include standard 3+1, 6, or 12 channel, or Cabrera 3+1, 6, or 12 channel in automatic mode, and 3, 6, or 12 channel rhythm strip printing. During rhythm strip printing user can toggle between the various 3 or 6 channels to print. The device can operate on battery or line power.
The ELI 230 includes:
• Acquisition module
• Hospital-grade power cord
• 1 pack paper (210mm roll paper)
• User manual CD
• Accessory starter kit
Intended Use
The ELI 230 is a multi-channel electrocardiograph product used for acquiring, viewing and printing resting ECG’s. The ELI 230 is a 12-channel diagnostic electrocardiograph intended for recording and printing ECG’s of adult and pediatric patients. The device is not intended to be used as a vital signs physiological monitor. The ELI 230 is intended to be used by a licensed health care practitioner in a hospital or clinical setting. It is designed to be used for acquiring, viewing and printing resting ECG’s. The ELI 230 is a standard 12-lead, electrocardiograph that is intended to be used with the Welch Allyn Wireless Acquisition Module (WAM) or Welch Allyn Acquisition Module (AM12) patient cables.
Indications for Use
• The ELI 230 Electrocardiograph is indicated for use to acquire, analyze, display and print electrocardiograms.
• The device is indicated for use for patients of any age, diseased or non-diseased.
• The device is indicated for use to provide interpretation of the data for consideration by a physician.
• The interpretations of ECG offered by the device are only significant when used in conjunction with a physician overread as well as consideration of all other relevant patient data.
• The device is indicated for use in a clinical setting, by qualified medical professionals, properly trained for ECG monitoring and use of the system. The personnel must be experienced in cardiovascular problematic situations and emergency procedures or pathologies related to cardiac involvements. It is not intended as a sole means of
diagnosis.
• The device is not intended to be used as a vital signs physiological monitor.
• The cardiac data and analysis provided is reviewed, confirmed, and used by trained medical personnel in the diagnosis of patients with various rhythm patterns.
Precautions
• Turn off the device before inspecting or cleaning.
• Do not immerse the device in water.
• Do not use organic solvents, ammonia-based solutions, or abrasive cleaning agents which may damage equipment surfaces.
Inspection
Inspect your equipment daily prior to operation. If you notice anything that requires repair, contact an authorized service person to make the repairs.
• Verify that all cords and connectors are securely seated.
• Check the case and chassis for any visible damage.
• Inspect cords and connectors for any visible damage.
• Inspect keys and controls for proper function and appearance.
Cleaning and Disinfecting the ELI 230
Disinfecting agents
The ELI 230 is compatible with the following disinfectants:
• Clorox Healthcare® Bleach Germicidal Wipes (use according to instructions on product label), or
• a soft, lint-free cloth dampened with a solution of sodium hypochlorite (10% household bleach and water solution) minimum 1:500 dilution (minimum 100 ppm free chlorine) and maximum 1:10 dilution as recommended by the APIC Guidelines for Selection and Use of Disinfectants.
Cleaning
To clean the ELI 230:
1. Disconnect the power source.
2. Remove cables and lead wires from device before cleaning.
3. Thoroughly wipe the surface of the ELI 230 with a clean, lint-free cloth dampened with a mild detergent and water for general cleaning or use one of the above recommended agents for disinfection.
4. Dry the device with a clean, soft, dry, lint-free cloth
WARNING:
Prevent liquid from penetrating the device and do not attempt to clean/disinfect the device or patient cables by submerging into a liquid, autoclaving, or steam cleaning.
-
Do not expose cables to strong ultra-violet radiation.
-
Do not sterilize the device or lead wires with Ethylene Oxide (EtO) gas.
-
Do not immerse cable ends or lead wires; immersion can cause metal corrosion. Use caution with excess liquid as contact with metal parts may cause corrosion.
-
Do not use excessive drying techniques such as forced heat.
-
Improper cleaning products and processes can damage the device, produce brittle lead wires and cables, corrode the metal, and void the warranty. Use care and proper procedure whenever cleaning or maintaining the device.
Disposal
Disposal must be in accordance with the following steps:
1. Follow cleaning and disinfection instructions per instructions in this user manual section.
2. Delete all existing data related to patients/hospital/clinic/doctor. Data backup may be performed prior to
deletion.
3. Segregate material in preparation for the recycling process
• Components are to be disassembled and recycled based on type of material
o Plastic to be recycled as plastic waste
o Metal to be recycled as Metals
• Includes loose components containing more than 90% metal by weight
• Includes screws and fasteners
o Electronic components, including the power cord, to be disassembled and recycled as Waste of Electrical and Electronic Equipment (WEEE)
o Batteries to be dismantled form the device and recycled as per WEEE
Users must adhere to all federal, state, regional, and/or local laws and regulations as it pertains to the safe disposal of medical devices and accessories. If in doubt, the user of the device shall first contact Hillrom Technical Support for guidance on safe disposal protocols.
ELECTROMAGNETIC COMPATIBILITY (EMC)
Electromagnetic compatibility with surrounding devices should be assessed when using the device.
An electronic device can either generate or receive electromagnetic interference. Testing for electromagnetic compatibility (EMC) has been performed on the device according to the international standard for EMC for medical devices (IEC 60601-1-2). This IEC standard has been adopted in Europe as the European Norm (EN 60601-1-2).
The device should not be used adjacent to, or stacked on top of other equipment. If the device must be used adjacent to or stacked on top of other equipment, verify that the device operates in an acceptable manner in the configuration in which it will be used.
Fixed, portable, and mobile radio frequency communications equipment can affect the performance of medical equipment. See appropriate EMC table for recommended separation distances between the radio equipment and the device.
The use of accessories, transducers, and cables other than those specified by Welch Allyn, may result in increased emissions or decreased immunity of the equipment.
ELI 230 Specifications
Radio specifications and certification information for the Wireless Acquisition Module (WAM) and USB Transceiver Key (UTK), can be found in the WAM user manual.
AM12 Specifications